![Thymoglobulin® [Anti-thymocyte Globulin (Rabbit)] Thymoglobulin® [Anti-thymocyte Globulin (Rabbit)] logo](/Images/mobile/thymo-logo.png)
- Important Safety
Information - Prescribing
Information - Medical
Information - For U.S. Healthcare Professionals Only
![Thymoglobulin® [Anti-thymocyte Globulin (Rabbit)] Thymoglobulin® [Anti-thymocyte Globulin (Rabbit)] logo](/Images/mobile/thymo-logo.png)
|
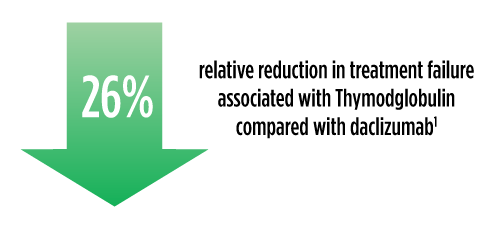
Efficacy: Thymoglobulin® Offers Proven, Proactive Protection Against Acute Rejection in Kidney Transplant Jump to Safety Information |
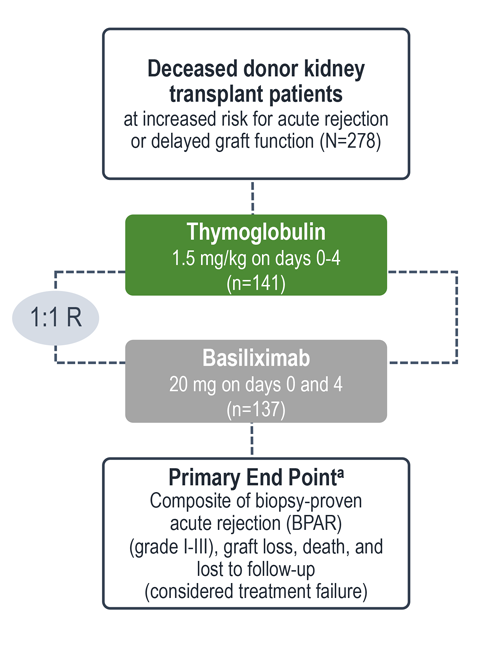
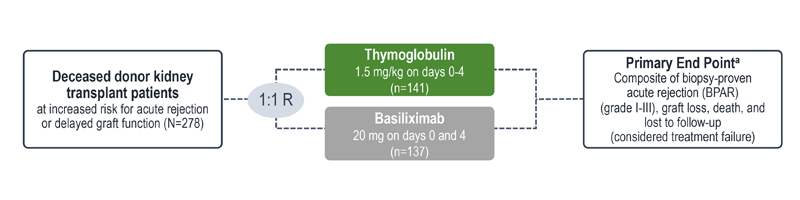
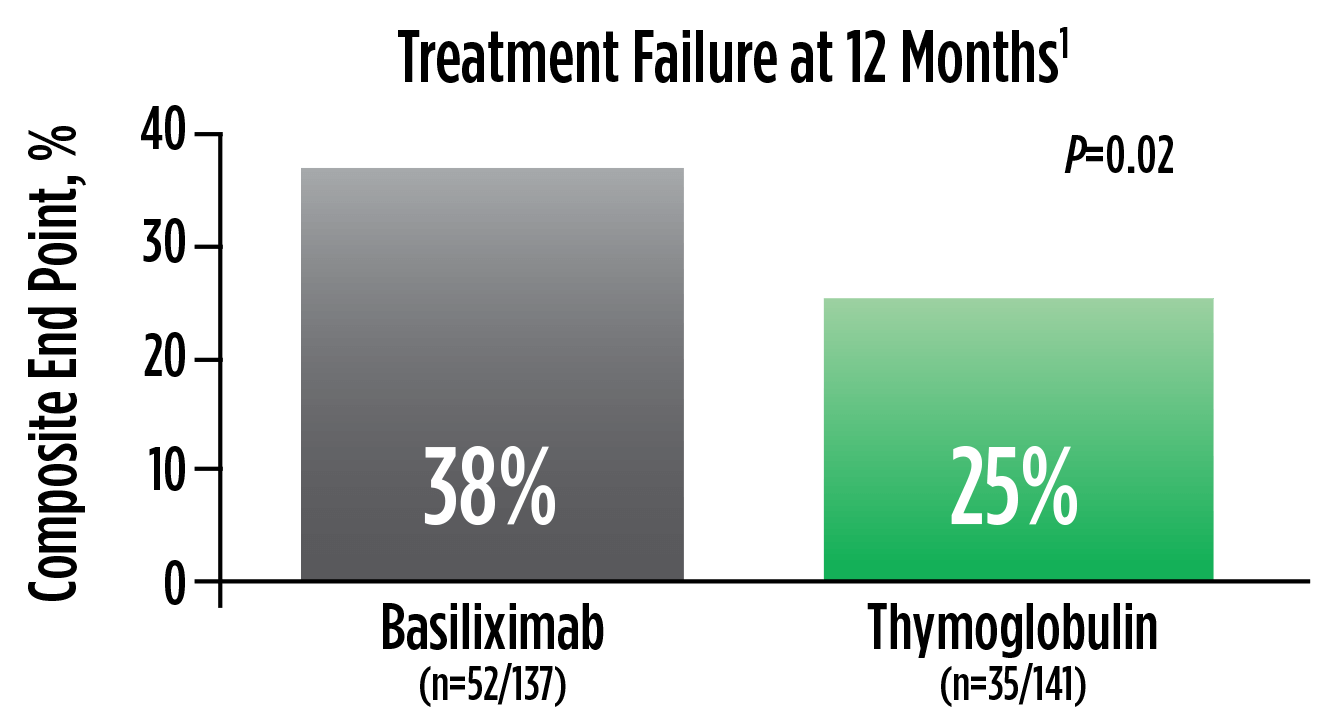
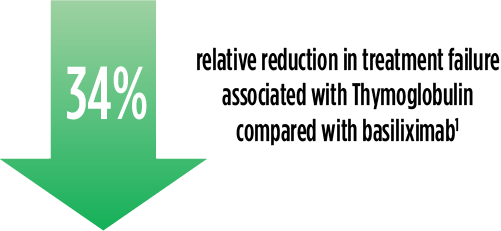
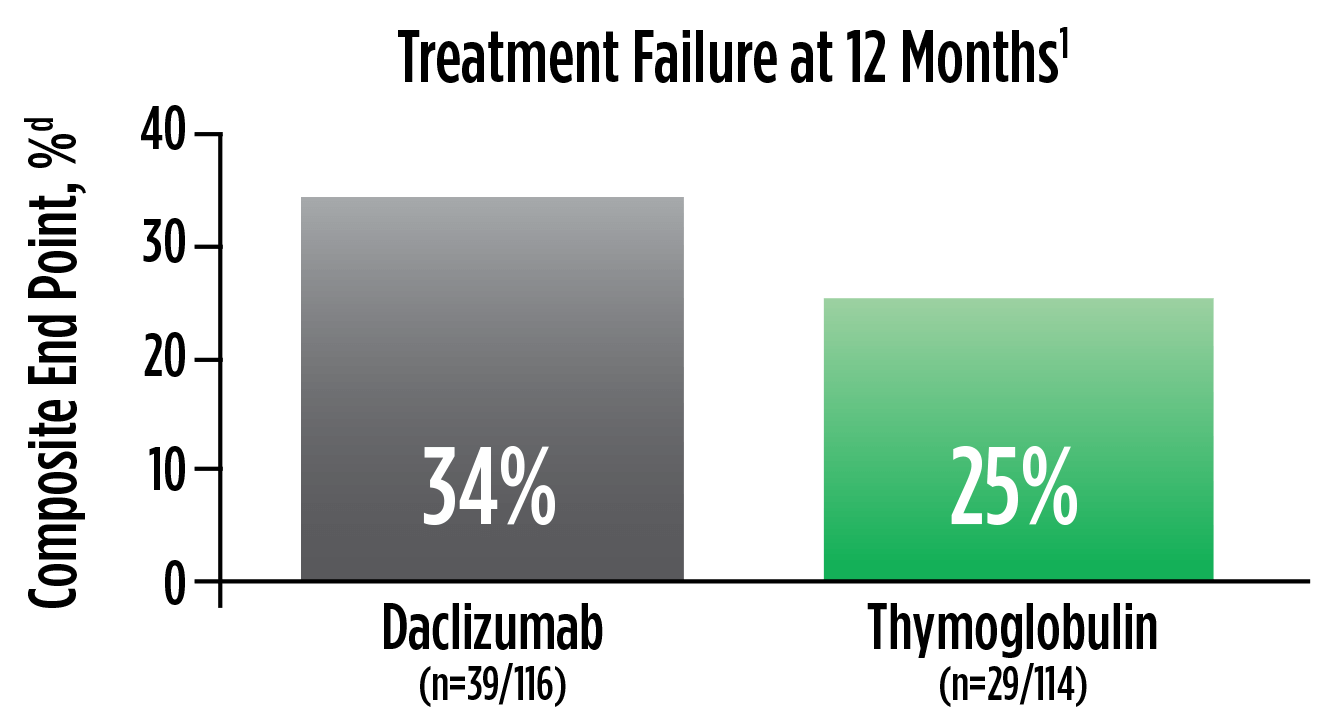
Treatment failure was based on the composite end point of BPAR, graft loss, death, or lost to follow-up within 12 months.1
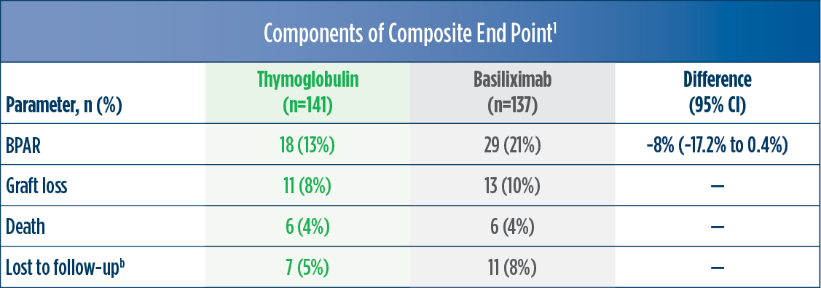

| a | The original primary end point of the trial published by Brennan et al was a composite of the first occurrence of BPAR, DGF, graft loss, or death.2 The FDA filing used a new composite end point, which removed DGF and included lost to follow-up, accounting for differences in the Brennan et al data compared with the Thymoglobulin label.3 The composite end point is defined as the occurrence of any of the following: BPAR (grade I-III), graft loss, death, or lost to follow-up. A patient can be counted in more than 1 category with the exception of lost to follow-up.1 |
| b | Lost to follow-up was defined as not having BPAR, graft loss, or death within 12 months post transplantation, and last visit date was prior to the lower bound of 12-month window (12 months ± 30 days after transplantation).1 |
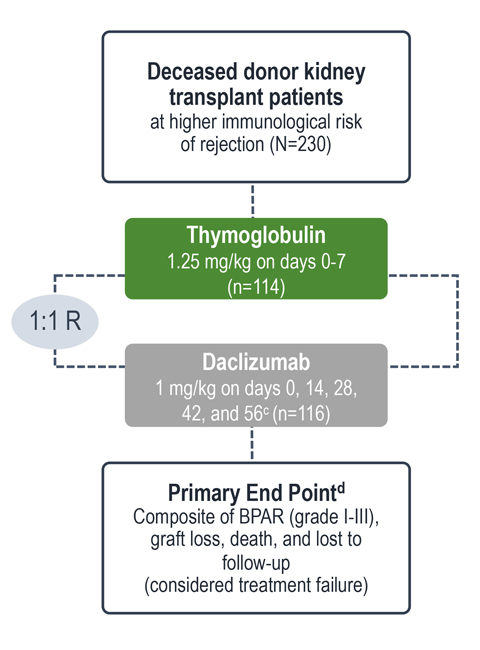
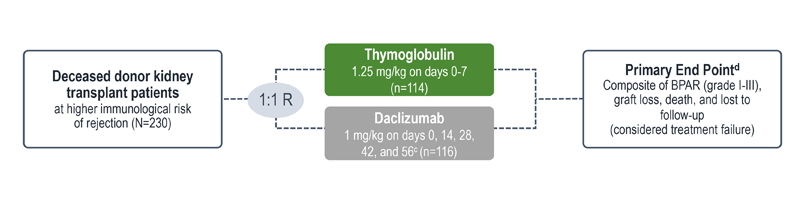
Treatment failure was based on the composite end point of BPAR, graft loss, death, or lost to follow-up within 12 months.1
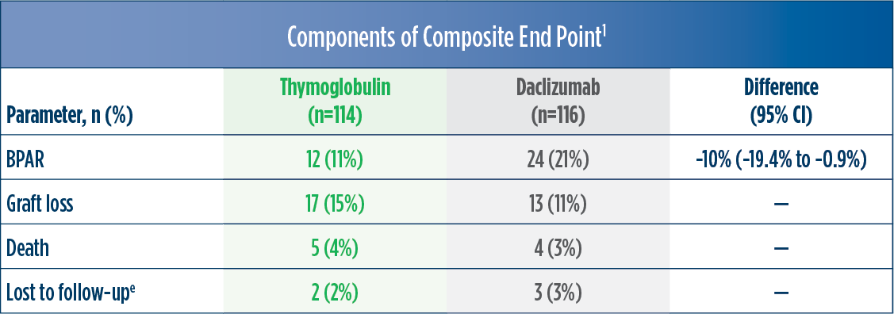

| c | Maximum dose of 100 mg.1 |
| d | The original primary end point of the trial published by Noël et al was BPAR within 1 year.4 The FDA filing used a common composite end point that included BPAR (grade I-III), graft loss, death, or lost to follow-up, which was included in the Thymoglobulin prescribing information. Different end points account for the differences in the treatment failure rate between the Noël publication and the Thymoglobulin prescribing information.3 |
| e | Lost to follow-up was defined as not having BPAR, graft loss, or death within 12 months post transplantation, and last visit date was prior to the lower bound of 12-month window (12 months ± 30 days post transplantation).1 |
|
Thymoglobulin Adverse Reactions and Laboratory Abnormalities1 Jump to Efficacy Information |
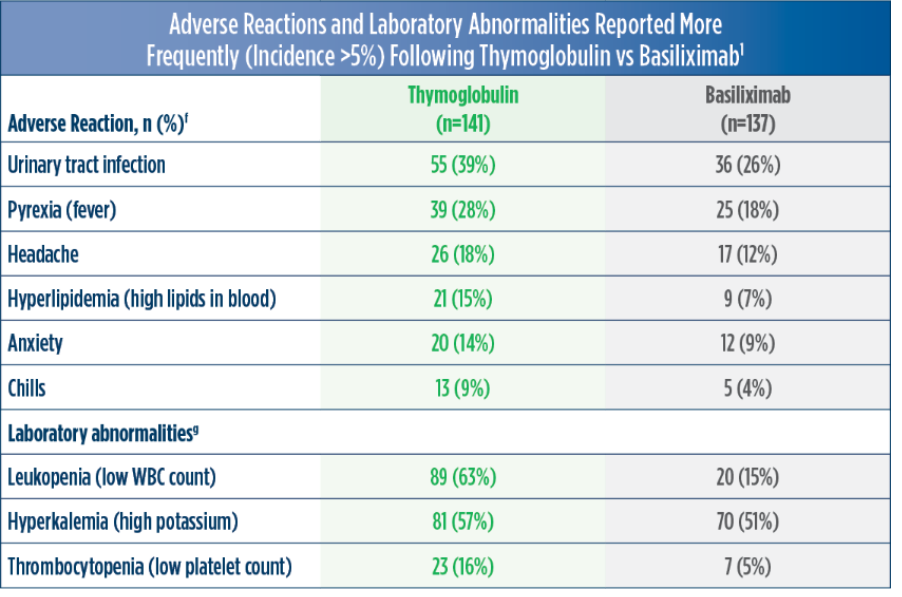
| f | Adverse reactions are TEAEs reported as related in at least 1 patient.1 |
| g | Leukopenia: WBC <3,000 cells /mm3; hyperkalemia: blood potassium ≥5.5 mmol /L; thrombocytopenia: platelet count <75,000 cells/mm3.1 |
Please see additional Important Safety Information below and click here for full Prescribing Information including Boxed WARNING.
WARNING: IMMUNOSUPPRESSION. Thymoglobulin should only be used by physicians experienced in immunosuppressive therapy in transplantation.
WARNING: IMMUNOSUPPRESSION. Thymoglobulin should only be used by physicians experienced in immunosuppressive therapy in transplantation.
Click here for full Prescribing Information including Boxed WARNING.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
Abbreviations: DGF, delayed graft function; FDA, Food and Drug Administration; MMF; Mycophenolate mofetil; TEAE, treatment-emergent adverse event; WBC, white blood cell.
References: